BACKGROUND
Sickle cell disease (SCD) is a devastating inherited disease process that greatly impairs quality of life and lifespan. It is a common genetic hemoglobinopathy that is vulnerable to hypoxemia due to the sickling property of red blood cells. Desaturation of the hemoglobin S (HbS) molecule leads to the polymerization of HbS, causing the blockage of microvasculature, oxidative stress, and endothelial damage. The clinical sequelae of SCD include vaso-occlusive crisis, hemolytic episodes, and end-organ damage. Obstructive sleep apnea (OSA) is a common but underdiagnosed condition associated with nocturnal hypoxemia due to intermittent upper airway closure. Repetitive episodes of hypoxemia can cause nocturnal sympathetic activation, selectively activate vascular inflammatory pathways leading to endothelial dysfunction, and can impact cardiac output to predispose patients to ischemia. The impact of this pathophysiology on SCD remains unclear and the literature reviews evaluating the relationship of SCD and OSA are scarce.
METHODS
We performed a retrospective analysis of the Nationwide Inpatient Sample data in adult patients (age greater than 18 years) hospitalized with SCD as the primary diagnosis in 2020 using ICD-10-CM codes. The NIS is the largest all‐payer publicly accessible inpatient healthcare database in the United States. We compared the SCD cohort of 164,990 patients to another consisting of SCD with associated secondary diagnosis of OSA, which consisted of 32,998 patients. The Pearson chi‐square test and Student's t test were used to assess categorical and continuous variables, respectively.
RESULTS
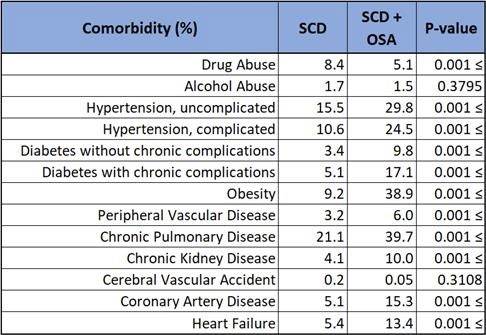
Data revealed that patients diagnosed with SCD and OSA had a statistically significant (p < 0.001) greater incidence of comorbidities compared to SCD alone. These included uncomplicated hypertension (29.8 vs 15.5%), complicated hypertension (24.5 vs 10.6%), uncomplicated diabetes mellitus (9.8 vs 3.4%), complicated diabetes mellitus (17.1 vs 5.1%), obesity (38.9 vs 9.2%), peripheral vascular disease (6.0 vs 3.2%), chronic pulmonary disease (39.7 vs 21.1%), chronic kidney disease (10.0 vs 4.1%), coronary artery disease (15.3 vs 5.1%), and heart failure (13.4 vs 5.4%). Illicit drug use was higher (8.4%) in SCD only compared to SCD with OSA (5.1%).
CONCLUSION
In this study, co-diagnoses of SCD and OSA revealed a greater likelihood of having other comorbidities, typically 2-3 fold. A striking finding is the increased prevalence of complicated hypertension and diabetes mellitus which are known to give way to vasculopathies, a sequela that is unfavorable in patients with SCD. Considering the clinical implications of these comorbidities and concern for overall underdiagnosis of OSA, it may prove beneficial to lower the threshold for OSA screening in patients with SCD who exhibit hypertension, diabetes, obesity, and other disease processes associated with OSA. Furthermore, should OSA screening in patients with SCD prove favorable, a prospective study evaluating outcomes, such as number of hospitalizations due to sickle cell crisis or acute chest syndrome, of patients diagnosed with OSA and subsequently treated with therapeutic measures such as nocturnal continuous positive airway pressure. Likewise, patients with established co-diagnosis of SCD and OSA should have diligent surveillance and treatment of these aforementioned comorbidities.
Disclosures
No relevant conflicts of interest to declare.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal